Abstract
Introduction
Sickle cell disease (SCD) is an autosomal recessive disorder associated with frequent pain and organ dysfunction. Approximately 33% of men with SCD have experienced at least one priapism episode. A priapism episode is often associated temporally with multiple events that may last hours to days without remitting and can result in erectile dysfunction, penile scarring, and sexual dysfunctions. Preclinical studies in transgenic SCD mice demonstrate that priapism occurs in the setting of relative nitric oxide (NO) deficiency due to chronic hemolysis. Based on the biological basis for priapism in SCD, we postulate that hydroxyurea, a nitric oxide (NO) donor, in combination with tadalafil, a phosphodiesterase-5 (PDE-5) inhibitor will restore normal homeostasis between voluntary erection and detumescence and decrease the incidence rate of priapism.
Methods
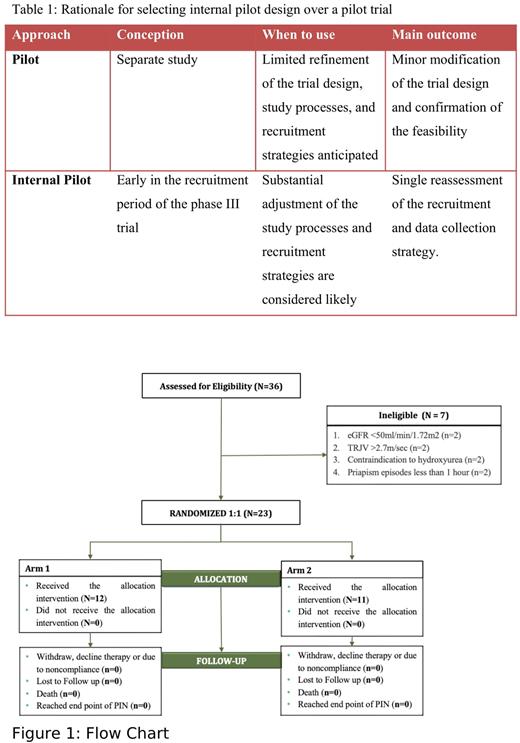
We designed a randomized controlled, double-blind internal pilot trial to prevent recurrent ischemic priapism in men with SCD. Participants will be randomly allocated to two arms in a 1:1 ratio: hydroxyurea 20 mg/kg/day and tadalafil 5 mg every morning or hydroxyurea 20 mg/kg/day and placebo. The inclusion criteria consist of participants with sickle cell anemia (HbSS/SßThal0), ages 18-40 years, who must have had at least 3 episodes of priapism (each lasting ≥1 hour) in the preceding 12 months. The exclusion criteria include decreased estimated glomerular filtration rate (<50 ml/min/1.72m2), high tricuspid regurgitation velocity (>2.7 m/sec), liver cirrhosis, and contraindication to PDE-5 inhibitors or hydroxyurea. The trial has a screening phase of 2-6 weeks (where priapism events occurring in this period are recorded) and a treatment phase (12 months). A strategy of daily text messaging is used to track priapism recurrences, whereas a paper diary is kept as a backup. The participants are seen monthly for study follow-up and drug adherence assessment. An independent data monitoring safety committee (DSMB) and medical monitors review the trial participants, procedures, and processes for safety. For a sample size of 100 and a baseline priapism rate of 11.6 events per year (estimates from observational data),5 and a mean follow-up of 1 year, we will have 99.9% power to detect a 33% reduction in the priapism rate using Poisson regression with an alpha of 0.05. We selected an effect size (33%) that was approximately the mid-point between the three FDA-approved therapies for the reduction in vaso-occlusive pain rate: crizanlizumab (45%), hydroxyurea (44%), and L-glutamine (25%).
Given the treatment effect for both arms is unknown, we chose an internal pilot trial design to allow for anticipated modifications of the study processes and recruitment strategies before the phase III trial (see Table 1). We planned an interim analysis to be conducted when approximately 50 participants have been followed for one year. During the interim analysis, assessment for efficacy and futility will be used to refine the final sample size based on the efficacy estimate. A recommendation to stop the trial for futility (based on priapism rates) will require a conditional power below 40% under the observed efficacy trend, with a two-sided type I error of <5%. Due to the potential that tadalafil is associated with an increased pain rate, an additional early stopping rule is based on the 95% confidence interval (CI) of the 1-year acute pain incidence rate and mortality rate ratios between the two arms. The trial will be paused and evaluated if the upper boundary of 95% CI is > 1.17. The 1.17, the inverse of 0.85, was chosen as a very conservative threshold based on the often-used FDA threshold of non-inferiority.
The trial is conducted at Aminu Kano Teaching Hospital (AKTH), and the protocol has been approved by the institutional review boards of both Vanderbilt University Medical Center and AKTH.
Results
The trial was opened on 13th April 2022 and is ongoing (see Figure 1 for the trial progress).
Conclusion
This phase II trial (NCT05142254) seeks to identify effective secondary prevention for priapism (a troubling complication of SCD), which currently has no evidence-based medical treatment strategy.
Disclosures
Idris:Agios pharmaceuticals: Consultancy. Burnett:Novartis, Futura Medical, Myriad Genetics, Comphya: Consultancy, Research Funding. DeBaun:Novartis, GBT, Vertex, and Forma Pharmaceutical: Consultancy, Research Funding.
OffLabel Disclosure:
Tadalafil, a phosphodiesterase type 5 inhibitor (PDE-5), is licensed by FDA mainly for treatment of erectile dysfunction. Preclinical and limited clinical data showed that PDE-5 inhibitors could paradoxically reduce the incidence of recurrent ischemic priapism in individuals with sickle cell disease. Our group is conducting an internal pilot trial to demonstrate the evidence for using this class of drug for secondary prevention of priapism.
Author notes
Asterisk with author names denotes non-ASH members.